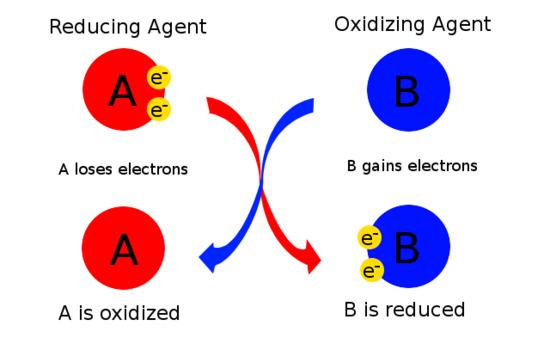
How to Tell Which Is the Best Reducing Agent
Click to see full answer. It has plenty of examples and practice problems for you.

Inorganic Chemistry Best Oxidizing And Reducing Agents Na Zn 2 Ba Ba 2 And Ag Chemistry Stack Exchange
All information related to How To Identify The Reducing Agent is displayed here.
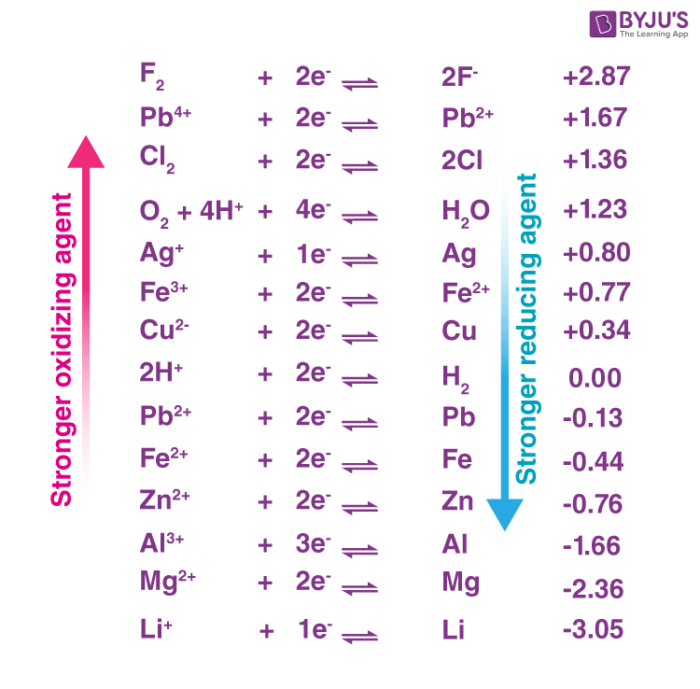
. B Nitrogen is being oxidized from an oxidation state of 0 to 1 so it is the reducing agent. The strongest oxidizing agent in the list is F2 followed by H2O2 and so on down to the weakest oxidizing agent Li. Good reducing agents include the active metals such as sodium magnesium aluminum and zinc which have relatively small ionization energies and low electro-negativities.
The reducing agent after losing electrons gets oxidized and also causes the opposite reactant to get. The best reducing agent is Na and the weakest is Cl-. The half reaction would be Li Li 1 e Oxidation of a Positive Oxidation State into a Higher Positive Oxidation State H 2 C 2 O 4 is also a good reducing agent.
Are weak reducing agents. The strongest reducing agents are the alkali metals Group 1 as they have low electronegativities and lose electrons very easily. So to identify an oxidizing agent simply look at the oxidation number of an atom before and after the reaction.
There are a few metals that do not just dissolve in any acid but need an acid such as HNO3 whose anion is a strong oxidising agent instead. The bigger the number the stronger the reducing. This chemistry video tutorial explains how to find the oxidizing agent and the reducing agent in a redox reaction.
Solution Verified by Toppr Correct option is A The more negative the value the stronger the reducing agent. This video tutorial shows you how to identify the oxidizing and reducing agent in a redox reaction. The best reducing agents are located at the bottom left of the periodic table low electronegativity and the best oxidizing agents are located at the top right of the periodic table high electronegativity excluding noble gases.
Popular reduction agents include potassium calcium barium sodium and magnesium metals as well as H-ion. If the oxidation number is greater in the product then it lost electrons and the substance was oxidized. Hydrogen is being oxidized from an oxidation state of 0 to 1 so it is the reducing agent.
A Nitrogen is being reduced from an oxidation state of 0 to -3 so it is the oxidizing agent. Metal hydrides such as NaH CaH 2 and LiAlH 4 which formally contain the H-ion are also good reducing agents. Fluorine chlorine iron etc.
As we move upwards from hydrogen in the electrochemical series then the strength of reducing agents decreases. While if we move downwards from hydrogen then the strength of reducing agents increases. The species at the top left have the greatest potential to be reduced so they are the strongest oxidizing agents.
Was this answer helpful. The metals of the s-block in the periodic table are said to be good. According to the chart it is unfavorable to oxidize Cl- aq or MnO s both have.
Characteristics of Reducing Agent Reducing agents tend to give away electrons. This eliminates choice B. According to the table Mg2 aq is an oxidizing agent because it has no electrons to lose and can gain two electrons to form the neutral metal.
Most metals are thus strong reducing agents. Some molecules such as. At top end of electrochemical series there is lithium which is the strongest reducing agent and at the bottom end of electrochemical series there is fluorine which is the weakest reducing agent or.
Good reducing agents tend to consist of atoms with a low electronegativity the ability of an atom or molecule to attract bonding electrons and species with relatively small ionization energies serve as good reducing agents. A reducing agent is a substance that causes another substance to reduce. The best reducing agent is the compound that is most favorably oxidized.
Is HCl a reducing agent. Lithium Li is a strong reducing agent because it readily loses an electron obtaining a 1 oxidation state. It explains how to determine which reacta.
To tell which is the strongest reducing agent one can change the sign of its respective reduction potential to make it oxidation potential. We can know the strength of reducing agents by electrochemical series as well. Corrosion requires an anode and cathode to take place.
Hence option A is correct. With just one click you can see the entire article information. From the table it can be seen that the standard reduction potential of Li is lowest so Li has the highest tendency to lose an electron and it is a best reducing agent.
H in HCl is the reactantreduced and HCl is the oxidizing agent. The oxidation state of the C atom is 3.

Comments
Post a Comment